The fabric obtained after weaving is known as grey fabric. It contains both natural as well as added impurities. In order to make the fabric suitable for dyeing and printing it is essential to remove the impurities present in grey fabric. The processes involved in the removal of these impurities are known as preparatory processes or fabric pre-treatment.
The chemical nature of both natural and added impurities present on grey fabric depends on the nature of fibre from which the fabric has been made. For example the chemical nature of added and natural impurities present on cotton would be different than those present on silk. Since cotton and silk are generally used in block printing, in this section the chemistry of added and natural impurities present on cotton and silk are discussed along with the chemistry involved in the removal of these impurities.
The purpose of pre-treatment is to remove added and natural impurities from the fabric. The nature of impurities depends on the nature of fibre from which the fabric has been made.
The added impurities present on cotton fabric are dust, oil stains and size
Dust
During weaving and during storage, the fabric attracts dust from the atmosphere. Since the dust is mechanically deposited it gets removed during the chemical processing operations used for the removal of other impurities. Therefore, no separate treatment is required for the removal of dust.
Oil stains
Oil stains are accidently formed on the fabric during the weaving operation due to negligence of worker during the oiling of loom parts. Such stains are removed during scouring operation under alkaline conditions.
Size
During weaving operation threads cross each other. The warp threads are under constant tension and undergo abrasion due to insertion of weft threads. For this reason the warp yarns are likely to break quite often during weaving; affecting the weaving productivity. In order to minimize the breaking of warp yarns and improve the weaving productivity it is essential to coat the warp yarns with a film forming polymer which gives a protective
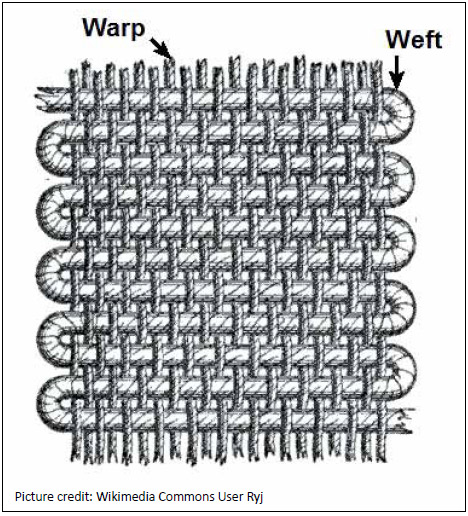
coating on yarn to prevent it due to abrasion during the insertion of weft yarn. The operation of coating the warp yarn with film forming polymer is known as sizing. This size is present as an added impurity on the grey fabric after weaving. Although sizing is essential or desirable during weaving, the presence of size is not desirable during dyeing and printing.
Why it is essential to remove sizing: If the size is not completely removed before dyeing or printing following undesirable effects would be obtained
- Poor water absorbency of fabric due inefficient removal of natural waxes present in cotton
- Non-uniform absorption of colour during dyeing and printing
- Low fastness of colour because part of the colour would be taken up by size which would be removed during washing of dyed/printed fabric. This would give false impression of low fastness property of colour.
Natural impurities in cotton
The presence of natural impurities would be revealed from the composition of cotton as shown in Table
Composition of cotton
| Constituent | % |
|---|---|
| Cellulose | 88 |
| Fats and Waxes | 0.5 |
| Pectin’s | 0.7 |
| Proteins | 1.1 |
| Colouring matter | 0.5 |
| Mineral Matter | 1.0 |
| Moisture | 8.0 |
It can be seen from the above Table that if we neglect the amount of moisture different types of natural impurities are present in cotton to an extent of 4% in total. These natural impurities are formed naturally during the growth of cotton fibres. The chemical nature of these impurities and why it is essential to remove them during pre treatment operation before dyeing and printing is outlined in Appendix 1.
Purpose of preparatory processes
The purpose of preparatory or pre-treatment processes is
- To remove natural and added impurities
- To impart certain desirable properties (water absorbency)
- To improve the appearance of fabric (whiteness)
- To make it suitable for subsequent processes like dyeing, printing finishing
- Removal impurities to the maximum extent with minimum effect on fabric strength. In case of cotton following chemical reactions are involved while removing the impurities
- Hydrolysis
- Oxidation
The added and natural impurities from grey fabric can be
removed by the following pre-treatment processes:
| Desizing | Scouring Alkali treatment | Souring Acid treatment | Bleaching |
|---|---|---|---|
| Removes: Starch, dust | Removes: Fatty substances, pectin’s and proteins | Removes: Mineral matter | Removes: Colouring matter |
The chemicals in pre-treatment are detailed in Appendix 2.
Chemistry of desizing
As mentioned earlier, desizing operation can be carried out by using the chemical reaction of hydrolysis or oxidation with the help of appropriate chemicals and process conditions. Hydrolysis of starch can be carried out by using:
- Rot steeping
- Strong acid like hydrochloric or sulphuric acid
- Amylase enzyme
Oxidation of starch can be carried out using oxidizing agents like
- Ammonium, sodium or potassium persulphate. Among these ammonium persulphate is most common
- Hydrogen peroxide
- Sodium hypochlorite or bleaching powder
Among the above oxidizing agents the use per sulphates is most common on industrial scale. Sodium hypochlorite or bleaching powder, though suitable and economical are not recommended because they are not environmentally friendly.
Oxidative desizing is carried out on large scale in organized textile mills. Therefore, this process is not recommended for block printers or small scale processors.
Hydrolytic desizing
Rot Steeping
In this process the grey fabric is soaked in water for 16-24 hours at room temperature. Fabric soaking is normally carried out in a cemented tank. The fabric is kept soaked in water by putting some weights so that it does not float above the water level. Otherwise the floated fabric layers will be exposed to air and will get dried due to which the starch removal from these exposed layers of fabric would not take place. After steeping for the required length of time the fabric is thoroughly washed with hot and cold water during which the hydrolyzed starch is removed.
Thus the steps involved in rot steeping are
- Soaking/saturation of fabric with water at room temperature
- Leaving the soaked fabric in water for 16-24 hours
- Washing with hot and cold water
- Drying (optional)
How starch hydrolysis takes place
In this process though no chemicals are used, the starch
present on the grey fabric acts as a food for the growth of micro-organisms (from atmosphere) on the surface of fabric soaked in water. The micro-organisms grown on the surface of grey fabric give out (excrete) amylase enzyme and the liberated enzyme is responsible for hydrolysis of starch making it water soluble.
Advantages
The process is simple and economical as no chemicals and energy is required.
Disadvantage
The process takes long time. However, main disadvantage of the process is that the growth of micro-organisms is not predictable since the growth of micro-organisms is a natural process. The micro-organism growth also depends on the atmospheric temperature. Thus in summer it would be more compared to in winter. Therefore the results obtained are not reproducible. For this reason the process though simple and economical it is not practiced now.
Acid desizing
In this method mineral acid like sulphuric acid or hydrochloric acid is used. The hydrolysis of starch takes place in presence of acid converting starch into water soluble products which are then removed by subsequent washing.
The desizing operation is carried out by following steps
- Soaking the fabric in sulphuric acid or hydrochloric acid solution (concentration 5g/l)
- Time of treatment 3-4 hours
- Temperature Room temperature
- Cold washing, washing with sodium carbonate solution 2 g/l), hot water washing
- Drying optional
Precautions
- Do not allow the fabric to float on the surface of water. At the exposed surface the concentration of acid would increase due to evaporation of water resulting in degradation of cotton at the exposed portions.
- Make sure that during washing operation acid is completely removed and the fabric shows neutral pH. This can be checked by adding a drop of universal indicator on the washed fabric and comparing the colour with the standard colour for neutral pH.
- The acid can also cause the degradation of cottan. Therefore, precautions in terms of concentration of acid, time of treatment and temperature must be taken to avoid loss of strength of cotton fabric.
Advantage
In addition to the removal of starch the process also
removes natural mineral matters present in cotton.
Enzyme desizing
Amylase enzymes are used for desizing of cotton. Amylase enzymes can be obtained from different sources such as
- Malt enzymes: These are obtained from fermented barley which is a grain
- Pancreatic enzymes: These are obtained from the pancreas (Digestive glands) of slaughtered animals.
- Bacterial enzymes: These are prepared from the growth of microorganisms under controlled conditions. Bacterial enzymes are preferred for desizing on industrial scale.
Desizing conditions
The desizing conditions for different types of amylase enzymes are summarized in the following Table
Enzyme desizing conditions
| Enzyme | pH | Temperature oC | Time hours |
|---|---|---|---|
| Malt | 4.5-5.5 (Slight acidic) | 55-65 | 2-3 |
| Pancreatic | 6.5-7 (Neutral) | 40-45 | 2-3 |
| Bacterial | 6.5-7 (Neutral) | 60-70 | 2-3 |
Enzyme desizing by small hand-printing units
The above precise conditions can be maintained when the desizing of cotton is carried out on industrial scale using suitable machine.
However, for hand-printers or small scale textile processors, the operation can be carried out in cement tank at neutral pH and at room temperature for 6-8 hours or preferably overnight. This is because since the operation is carried out at room temperature, the time of treatment must be increased.
Washing
After desizing the fabric must be washed thoroughly with
- Hot water once
- Cold water twice
Drying is optional, because the next operation is scouring which can be carried out by using wet desized and washed fabric.
Enzyme desizing on Jigger
Jigger is a common machine used for pre-treatment and dyeing operations. Compared to desizing in cement tanks, desizing on jigger is preferred because desirable conditions required can be maintained.
The conditions for desizing on jigger using bacterial enzyme are summarized below
| Enzyme concentration | 3-5 g/l |
| pH | 6.5-7 (Neutral) |
| Wetting agent(Non-ionic) | 1 g/l |
| Temperature | 60-70oC |
| Time | 2-3 hours |
| After treatment (on jigger) | |
| Cold wash | 2 rounds |
| Hot wash 80-90oC | 2 rounds |
| Cold wash | 1rounds |
| Drying is optional |
Desizing efficiency (Testing for the removal of starch) Iodine Test
At the end of desizing it is essential to test whether the starch is completely removed from the fabric or not. Complete removal of starch can be tested by iodine test. The steps involved in this test are
- Place a drop of iodine solution on the wet desized fabric (If the fabric is dried after desizing, it is suggested to wet out the dry fabric before putting drop of iodine solution)
- Spread the iodine solution on wet fabric by slight rubbing action
- Wait for 1 minute for the reaction to complete between residual starch and iodine
Observations
Observe the change in colour of iodine solution on the fabric. Depending on the change in colour the extent of removal of starch can be predicted qualitatively.
The change in colour is illustrated in the following picture
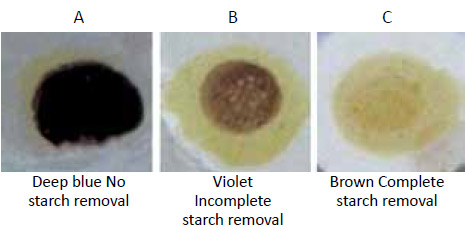
- Dark blue colour: No removal of starch. In this case desizing process must be repeated
- Light violet colour: Partial removal of starch. The fabric may be accepted for next process
- Light brown colour: Complete removal of starch. Most desirable. Desizing process has been carried out efficiently.
The conditions under which the block printers or small scale processors carry out the desizing operation it is difficult to remove starch completely to an extent of getting brown color in iodine test. Therefore, if the desized fabric shows light violet color in iodine test, the fabric may be accepted for the next operation of scouring and bleaching. It is anticipated that during the operations scouring and bleaching the residual starch would be removed. However, under no circumstances the fabric showing deep blue colour be accepted. It must be subjected to desizing operation again.
Reason for deep blue colour between starch and iodine
The exact reason for the change in colour of iodine solution brought in contact with starch is not known precisely. However, it is assumed that there is complex formation between iodine and starch and this complex has blue colour.
Preparation of iodine solution for test
- Dissolve 6 gm of potassium iodide in 100 ml distilled water
- Add 2 gm of iodine crystals to potassium iodide solution and stir with glass rod till iodine is completely dissolved
- Store the solution in dark amber coloured glass bottle with cap
- Put the iodine drop on the fabric with the help of dropper
Scouring
The scouring process consists of treatment of cotton fabric under alkaline conditions at high temperature. The process on small scale sector is also known as boiling.
Wet or dry fabric
After desizing the cotton fabric is subjected to scouring operation. The desized fabric can be used in wet condition or dry condition. The choice depends on the time gap between desizing and scouring. If the desized fabric is taken immediately for scouring then it may be in wet condition. However, if there is large time gap of 2 days and more, then desized fabric should be dried. If the wet cotton fabric is stored for long time then as in rot steeping, the microorganisms from atmosphere would grow on the wet fabric and leave black stains on fabric which are very difficult to remove during subsequent operations. Also the stored fabric would smell and there is possibility of fabric degradation.
Purpose of scouring
The impurities removed during scouring process are
- Natural impurities like fats, waxes, pectic substances, proteins, seed coats
- Added impurity like oil stains
The main purpose of scouring is to improve water absorbency of cotton fabric by the removal of fats and waxes. Good water absorbency is an essential requirement for uniform bleaching, dyeing and printing processes. The fabric appearance is also improved by the removal of seed coat fractions (kitties) adhering to the fabric surface.
Scouring chemistry
The chemistry behind the removal of impurities during scouring is summarized in the following table.
Chemistry of removal of impurities during scouring
| Impurity | Chemical reaction involved |
|---|---|
| Oils, fats and waxes | By the action of sodium hydroxide alkali these impurities are converted into water soluble form with the formation of soap. Therefore the chemical reaction is also known as saponification |
| Pectic substances | Converted into water soluble salts of pectic acid by reaction of sodium hydroxide |
| Proteins | Proteins are converted to water soluble amino acids |
| Lubricants and oil stains | Converted to water soluble products |
Scouring agents
- Alkali: Sodium hydroxide is the main alkali. However, other alkalis like sodium carbonate, sodium bicarbonate, trisodium phosphate, sodium silicate are used alone or in combination with each other depending on the need of the scouring process.
- Surfactants/wetting agents: These agents improve the wettability of fabric which is essential for penetration of chemicals for efficient effect on the impurities to be removed. Anionic or non-ionic wetting agents are recommended. The selected wetting agent/surfactant should be stable to alkali and the process conditions.
- Emulsifying agents (non-ionic): These agents keep the degraded impurities in suspended form to prevent their re-deposition on fabric. These agents facilitate the easy removal degraded impurities during subsequent washing.
- Sequestering agents: These are also known as metal chelating/complexing agents. They bind heavy metals like cu, Fe, Ca, Mg etc. present in water and thus help to minimize fabric degradation. Most common agent is EDTA (Ethylene Diamine Tetra Acetic acid). Many other trade products are available.
Typical recipe
The choice of recipe and the process conditions depend on various factors such as type of fabric to be scoured, equipments and other facilities available. Each processor has to standardize own recipe to get satisfactory results. Therefore, the following recipe may be taken only as a guideline.
| Sodium hydroxide (NaOH) | 3% |
| Sodium carbonate (Na2CO3) | 1% |
| Wetting agent | 0.2-0.4% |
| Sequestarant | 0.1-0.2% |
| Emulsifying agent | 0.2% |
The concentration of chemicals in the above recipe is expressed on the basis of weight of fabric (owf). For example if the weight of the fabric to be processed is 1kg the concentration of Sodium hydroxide (NaOH) is 30 gm. The concentration of other chemicals is calculated similarly.
Process conditions
- Volume of water: This is known as Material to Liquor ratio (M:L) This is the volume of water to be taken for the processing of given weight of the fabric. It varies depending on the equipment used for processing. Two types of equipments are most commonly used for scouring of cotton fabric
- Kier: In kier the volume of water could be 1:10. This means for 1 kg of fabric weight 10 litres of water is required
- Jigger: In jigger the volume of water could be 1:5. This means for 1 kg of fabric weight 5 litres of water is required.

2. Temperature: boiling
3. Time for the process
- In kier 4-6 hours
- In jigger 2-3 hours
4. After treatment:
- Cold water : Wash two times
- Hot water: Wash (70-80oC). Wash once
- Cold wash: One time
Acid wash or souring
This process is also known as souring. Some time acid wash using 1-2 g/l of hydrochloric or sulphuric acid is recommended for the removal of residual alkali. If acid wash is given, then precaution should be taken that the residual acid from the fabric is completely removed and the fabric pH is neutral. If the acid is not completely removed then there would be fabric degradation during subsequent drying operation.
If the bleaching process is carried our immediately after scouring there is no need of drying the scoured fabric. However, if the bleaching process is delayed then fabric should be dried.
Determination of scouring efficiency
The main purpose of scouring is to improve the water absorbency of fabric. This is tested by several tests such as
- Water drop absorption
- Sinking time test
- Capillary rise method
Among these, water drop absorption test is simplest and quick. Therefore this test is commonly used.
In this method a drop of water is placed on the dry scoured fabric. If the drop of water is absorbed within 3-5 seconds then it is considered that scouring operation is carried out efficiently.
If the drop of water floats on the fabric surface for a long time (more than 2 minutes) it indicates that the scouring is not done efficiently and there is a need to repeat the process.
Loss in fabric strength
Many times good water drop absorbency is achieved due to excessive scouring. Therefore, if the facility for fabric strength measurement is available then tensile or tear strength of the fabric must be tested and the strength results are compared with the strength of grey fabric. If loss in strength is more than 10-15% it indicates excessive scouring operation.
Bleaching
Bleaching operation is carried out to improve the whiteness of fabric. This is achieved by the process known as bleaching. During bleaching the natural colouring matters present in cotton are decomposed to colourless substances. The removal of these colouring matters helps to improve the whiteness of cotton fabric.
Purpose of bleaching:
- To produce white fabric by destroying colouring matter with minimum fibre degradation.
- To improve brightness of colour after dyeing or printing
- Further improvement of whiteness by treatment with optical brightening agents when the fabric is to be marketed as white
Bleaching agents
The chemicals used for improving the whiteness of fabric are known as bleaching agents. Although several bleaching agents are available, hydrogen peroxide is most popular for bleaching of cotton.
Properties of hydrogen peroxide
The important properties of hydrogen peroxide are summarized below
- Colourless liquid
- Corrosive to skin, dangerous to eyes
- Stable under acid pH
- Activated under alkaline conditions. Chemicals like sodium hydroxide, sodium carbonate, trisodium phosphate alone or in combination may used as alkali for activation.
- Decomposition in presence of alkali alone is very rapid resulting in uneven bleaching. Hence the use of stabilizer along with alkali is essential during bleaching.
Hydrogen peroxide stabilizer
Compounds which control the rate of decomposition of hydrogen peroxide under alkaline conditions are known as peroxide stabilizer. Sodium silicate is the most common and economical stabilizer. Commercially silicate and non-silicate based products are also available.
Typical bleaching equipment
Equipment
- Kier: On small scale kier can be used for bleaching of cotton with hydrogen peroxide. The most important precaution to be taken during bleaching in kier is that the inside walls of the kier must be thoroughly cemented so that the peroxide solution does not come in contact with iron from which the kier might have been constructed. If this precaution is not taken the iron walls of kier would act as catalyst for the rapid decomposition of hydrogen peroxide even in presence of stabilizer. This would result in uneven bleaching and also fabric degradation. Ideally a stainless steel kier must be used for peroxide bleaching.
In kier the bleaching process is carried out while the fabric is in rope form. Therefore the liquor circulation must be efficient to get uniform bleaching.
- Jigger: This is most suitable equipment as the commercial jiggers are made of stainless steel. Therefore there is no danger of rapid decomposition of hydrogen peroxide and fabric degradation. The other advantage of jigger is that the fabric is processed in open width form. Therefore the treatment is more uniform.
| Typical bleaching recipe | |
|---|---|
| Hydrogen peroxide (35 %) | 3-5% owf |
| Wetting agent | 0.1-0.5% owf |
| Sodium hydroxide (NaOH) | 0.3-0.8% owf |
| Sodium silicate | 2-3% owf |
| Magnesium sulphate (Epsom salt) | 0.5% owf |
owf means the concentrations are on the basis of weight of fabric.
Process
- Maintain the temperature between 80oC and 100oC
- Duration for the process: 60-120 minutes
- After the process is complete, drain the water; and,then rinse with hot and cold water
Bleaching Efficiency
Whiteness of bleached fabric: The bleaching efficiency can be tested by expressing the whiteness of bleached fabric in terms of whiteness index (or percentage reflectance). The whiteness index of 70% and above can be considered as acceptable whiteness.
For measurement of whiteness, spectrophotometer equipment is essential.
If a standard white fabric is available then whiteness of the bleached fabric can be compared with the standard white fabric visually. This test can be done in the absence of equipment. However, the test being visual it can be subjective. Therefore, instrumental test is recommended to avoid any discrepancy.
Strength measurement: The strength measurement (Tensile or tear strength) does not give idea about the whiteness, but it gives an idea whether the bleaching operation is carried out without fabric degradation. The loss in strength up to 10% compared to scoured fabric normally is acceptable. If Strength loss is beyond 10%, then bleaching recipe and conditions must be reviewed.
Optical brightening treatment
The whiteness of cotton fabric achieved during hydrogen peroxide bleaching is adequate if the fabric is subsequently dyed or printed. However, it is not adequate if the fabric is to be sold or marketed as white. For this purpose there is a need for further improvement of bleached fabric whiteness. This can be achieved by treating the bleached fabric with
- Bluing agent or
- Tinting dyes or
- Optical brightening agent
It must be remembered that the treatment with above agents is given only when the fabric is to be sold as white. If the fabric is to be dyed or printed then above treatment is not essential.
The improvement of whiteness of cotton fabric during H2O2 bleaching is a chemical process due to conversion of colouring matter into colourless water soluble products and their subsequent removal from the fabric.
On the other hand, the improvement of whiteness due to bluing agent, tinting agent or optical brightening agent is a physical process.
Mechanism of whiteness improvement
Bluing and tinting agent
These agents reflect the light in the blue region of visible spectrum and thus compensate the yellow tinge of white fabric. Thus when the bleached fabric is treated with these agents the amount of blue light reflected from the fabric surface is higher than the fabric that has only been bleached. Thus the treated fabric looks more white compared to only-bleached-fabric. However, in order to look the fabric white it is essential to use the minimum concentration as recommended by the manufacturers. If the concentration is higher than recommended then the fabric instead of looking white would look tinted.
Optical brightening agent (OBA)
These are also known as fluorescent whitening agent. The property of optical brightening agent is that it absorbs light in the ultraviolet region and reflect the absorbed light in the blue region of visible spectrum.
When bleached cotton fabric is treated with OBAs, there are two sources from which light is reflected from the fabric surface these are fabric surface itself and the amount of light absorbed in the ultraviolet region and reflected in the visible region by OBA. Thus the total amount of light reflected from the OBA treated fabric is higher than onlybleached- fabric. Thus OBA treated fabric looks whiter.
Application
Bluing agent
Bluing agent sometime also known as Neel. It is a blue pigment which gets dispersed in water. The bleached fabric is treated either in cement tank or jigger with 0.25-0.5 g/l concentration of bluing agent. The treatment is carried out at room temperature for 30 minutes. After the treatment the fabric is squeezed and dried in open-width form. There is no need of washing after treatment. Higher concentration of bluing agent should be avoided.
Tinting dyes
These are acid dyes having no affinity to cotton. They cause only tinting which is desirable for whiteness improvement. Readymade mixtures of tinting colors are available either in liquid or solid form. The manufacturers’ recommendations must be followed for whiteness improvement.
Optical Brightening Agent (OBA)
Bluing agent or tinting agents do not show affinity to cotton whereas optical brightening agents have affinity to cotton. Therefore they are also called as white or colorless dyes. The fabric is treated with 0.25-0.5% (owf) with OBA. Higher concentration should be avoided otherwise the whiteness would be lowered due to yellow tinge.
The treatment can be carried out at room temperature or at 60oC. There is no need of washing after OBA treatment.
OBA treatment during bleaching
Some OBAs are stable to hydrogen peroxide (H2O2); therefore the bleaching and OBA treatment can be carried out simultaneously. However, manufacturer’s recommendation must be followed for the choice of H2O2 stable OBA.
Leave a Reply